
Talk Test Treat Trace
Chapter 8: Improving access to testing and treatment of hepatitis B and C
In this chapter
Key points
- Direct acting antiviral agents (DAA) for the treatment of hepatitis C:
- have greater than 95 per cent cure rates
- can prevent or limit progression to cirrhosis and liver cancer
- can be given as a single daily dose over shorter treatment times and have fewer side-effects than older treatments, enabling improved adherence
- can be prescribed by medical and nurse practitioners through primary healthcare services, improving access to treatment.
- Regular monitoring and treatment of chronic hepatitis B can reduce the progress of liver damage.
- Despite advances in treatment, most people living with hepatitis B and C remain untreated and are not receiving appropriate management.
- Primary health services can help to address gaps in testing and management and improve access to treatment of hepatitis B and C in a variety of ways:
- Ensure services are accessible and acceptable for often marginalised clients.
- Provide community education highlighting easy access to new and effective treatments for hepatitis C.
- Support training to ensure all staff understand the benefits and criteria for accessing new hepatitis C treatments.
- Support training to enable practitioners to increase their confidence and competence with regard to management and prescribing of DAA.
- Strengthen recall systems to reduce loss to follow-up.
- Conduct clinical audits to identify those lost to follow-up, to re-engage them with health services and increase access to treatment.
Background
Hepatitis B and hepatitis C remain common infections that can lead to serious consequences, such as cirrhosis and hepatocellular (liver) cancer (HCC). Aboriginal people are over-represented with regard to rates of infection and burden of ill health. Of the 10,537 new hepatitis C infections notified in Australia in 2017, 69 per cent were among men and 11 per cent were among Aboriginal people. The rates of new infections have also increased significantly since 2012, possibly due to a combination of factors such as higher unsafe injecting rates, high rates of incarceration and increased case detection.
The availability of new DAA, which have greater than 95 per cent cure rates for hepatitis C, can prevent or limit the progression of liver disease and make the elimination of hepatitis C in the community achievable. In contrast to older treatments, DAA have fewer side-effects and can be given as a single daily dose over a shorter time, leading to improved adherence. In 2017, among an estimated 182,144 people living with chronic hepatitis C in Australia, 80 per cent had been diagnosed but only 47 per cent of those people had an HCV ribonucleic acid (RNA) blood test to confirm the diagnosis of chronic infection. Among people confirmed as having a chronic infection, only 31 per cent received DAA treatment, among whom 95 per cent were cured.
Among about 233,947 people living with chronic hepatitis B in Australia in 2017, it is estimated that only 64 per cent have been diagnosed, only 18 per cent are receiving regular guideline-based care and only 8 per cent have received antiviral treatment, highlighting significant gaps in testing and management. While the treatment of chronic hepatitis B is not as straightforward or effective as with hepatitis C, qualified s100 community prescribers can prescribe hepatitis B treatment which, together with regular six to 12 month monitoring through primary health services, can reduce the progress of liver damage and loss of liver function.
Medical and nurse practitioners can prescribe DAA for hepatitis C, enabling treatment and support to be given through primary health services, increasing access for priority populations. The challenge for health services is to ensure barriers to access are reduced through providing appropriate information to clients, active case finding of those lost to follow-up and supporting staff training to enable provision of effective management.
Transmission of hepatitis A, B and C
Hepatitis A is transmitted by the faecal–oral route, usually after ingestion of contaminated food and water or through oral–anal sex. Hepatitis A can be a serious infection, especially if acquired during pregnancy, but it never leads to chronic infection or reinfection. People become immune to hepatitis A following the resolution of infection or after immunisation.
Hepatitis B is transmitted through blood and body fluids, with most cases a result of mother to baby transmission, unsafe injecting or unprotected sex. Chronic infection occurs in up to 90 per cent of people infected at birth in contrast to up to 10 per cent infected as adults. Immunity against reinfection occurs following resolution of an acute infection or after immunisation.
Hepatitis C is transmitted mainly by blood to blood exposure with about 90 per cent of new infections among people with a history of injecting drug use. A history of imprisonment is an independent risk factor for hepatitis C, with prevalence of 30 to 40 per cent among all prisoners and higher rates of 50 to 60 per cent among female prisoners. The risk of sexual transmission is low, but increases among people with human immunodeficiency virus (HIV) or high risk sexual practices.
Table 4. Hepatitis A, B and C viruses
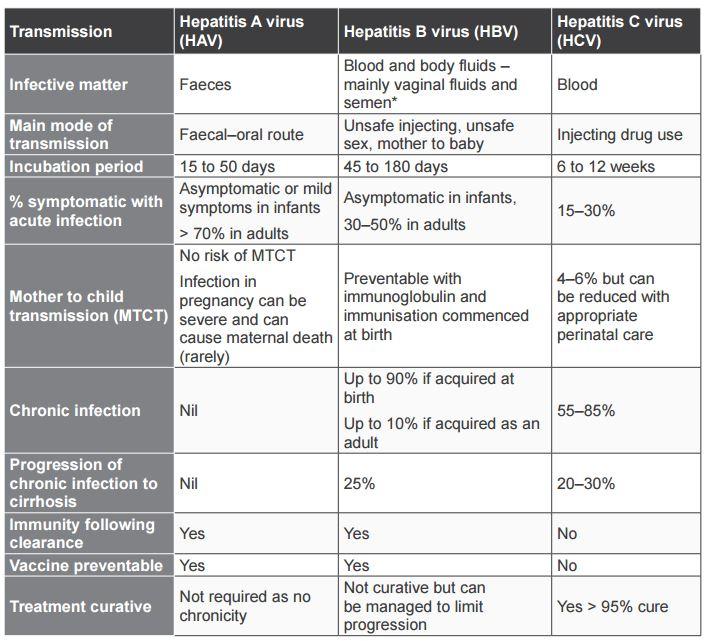
* Hepatitis B is found in small amounts in tears, saliva and breast milk but not in levels considered high enough to cause transmission.
Natural history of hepatitis A, B and C
Hepatitis B and hepatitis C can both cause chronic infections, which may progress to chronic liver disease, cirrhosis and HCC liver cancer. The progression to cirrhosis increases in the presence of other factors, including co-infection with hepatitis B and hepatitis C, HIV, obesity, insulin resistance and alcohol intake > 40 g/day.
Hepatitis C can resolve naturally within six months from the time of infection; however, most people (55 to 85 per cent) do not clear the virus, leading to chronic infection. Between 5 and 10 per cent of people with a chronic infection will develop cirrhosis within 20 years and a further 10 to 15 per cent after 40 years. Each year, 3 to 5 per cent of people with cirrhosis will develop HCC.
Among people with chronic hepatitis B, up to 25 per cent will progress to cirrhosis and HCC. In contrast to hepatitis B, people who clear hepatitis C naturally or following treatment do not develop immunity and can be reinfected if re-exposed to the virus.
Mother to child transmission
Hepatitis B and hepatitis C can be transmitted from mother to child although transmission of hepatitis B is now rare in Australia due to the universal immunisation of neonates (newborns) and appropriate management of mothers and their babies. Pregnant women should always be managed in conjunction with specialists experienced in managing hepatitis B and hepatitis C.
Transmission of hepatitis C from mother to baby is about 4 to 6 per cent and increases in the presence of HIV. Currently, treatment with DAA cannot be given during pregnancy, highlighting the need for increasing access to testing and treatment for women at risk prior to pregnancy. Babies born to mothers with hepatitis C should have an HCV RNA test at eight weeks and again 4 to 6 weeks later to confirm or exclude hepatitis C as well as an anti-HCV antibody test (HCV Ab) at 18 months.
Immunisation
Hepatitis A and hepatitis B are vaccine preventable infections, but there is no vaccine currently available for hepatitis C. Ensure those at risk for hepatitis are fully immunised for both hepatitis A and hepatitis B (if not already immune) and that their immunisation history and immune status are appropriately documented in their medical records.
Hepatitis C: risk factors, testing and treatment
Who and when to test for hepatitis C
Be familiar with the different types of tests for hepatitis C, when to use them and how to interpret results in order to provide clients with appropriate information regarding test results. Information regarding the interpretation of these and other tests is outlined below.
Most people with a past history of risk for transmission, rather than an ongoing risk, only need to be screened once for hepatitis C. With regard to screening, the HCV Ab test should be used among people who have either never been tested or who have never had a reactive HCV Ab test result. As the HCV Ab test will remain reactive for life, it cannot be used to check for re-infection. The HCV RNA test needs to be used to check for re-infection among people at ongoing risk who have previously had a reactive HCV Ab but cleared the infection.
People with any indication of liver disease should be tested for hepatitis C. Liver disease may result from a combination of infective and other causes, and risks for one blood-borne virus (BBV) may indicate risk for others. Even if one cause of liver disease, such as alcohol misuse, is evident, all people with liver disease should be tested for possible infective causes (hepatitis A, hepatitis B and hepatitis C at a minimum) and HIV. Tests should be taken among people who present with:
- any signs or symptoms of liver disease
- abnormal liver function tests (LFTs), in particular elevated enzymes (alanine aminotransferase or ALT)
- liver abnormalities identified on imaging.
People should also be tested for hepatitis C at least once if they have ever had a risk factor for transmission. More frequent screening may be needed among people with ongoing risk factors. People who may have been at risk for hepatitis C and who should be tested include those who have:
- ever injected drugs
- ever been in prison
- ever had any unsafe tattooing, skin piercings or scarification
- had a needle stick injury (occupational or non-occupational exposure)
- received organ, tissue or blood products prior to the introduction of hepatitis C screening in Australia in 1990 (or at any time in other countries)
- hepatitis B or HIV
- sexual practices that put them at risk, such as traumatic sexual practices, group sex or unprotected anal intercourse, particularly in the presence of HIV or sexually transmitted infections (STIs).
Other people who should be tested are:
- sexual partners of people with hepatitis C
- children born to mothers with hepatitis C
- people born in high prevalence regions such as Africa, the Middle East, the Mediterranean, Eastern Europe, and South Asia.
In addition to those with specific risk factors, all pregnant women should be tested for hepatitis C at their first antenatal visit and again in the third trimester if at higher risk.
Testing for hepatitis C among people with ongoing risks
Screening for hepatitis C should be done at least annually and more frequently (3 to 6 monthly) among people with ongoing risks for transmission such as:
- People who inject drugs (PWID):
- annually among those with safe, sterile injecting practices
- 3 to 6 monthly among those with unsafe, unsterile injecting practices
- sexual partners of people with hepatitis C with high risk sexual practices
- HIV positive people.
If a person has ever had a reactive HCV Ab test, it will remain reactive for life and therefore cannot be used to check for reinfection among people who have been exposed but cleared the infection in the past. In this case, an HCV RNA test is used to check for reinfection. Screening for hepatitis C should be done using the following test:
- HCV Ab – if never tested or previous HCV Ab was non-reactive
- HCV RNA – if they have ever had a reactive HCV Ab test.
An HCV RNA test should also be taken in the following circumstances:
- following an initial reactive HCV Ab test to determine cleared or chronic infection
- repeat in six months after an acute infection to determine whether the virus has been cleared or not
- repeat in six months if reactive and there is no history of a previous HCV RNA test to confirm chronic infection
- at the completion of DAA therapy to measure response to treatment
- annually as a screening test to check for reinfection among people who have ever had a reactive HCV Ab test and have ongoing risks for reinfection.
The HCV viral load (how much of the hepatitis C virus is in the blood) can be measured. While not clearly associated with the likelihood of clearing the infection, measuring the viral load is usually done before treatment and may identify people eligible for shorter treatment regimens.
Hepatitis C: interpretation of test results
Be familiar with the different types of tests for hepatitis C, when to use them and how to interpret results in order to provide clients with appropriate information regarding test results.
The HCV Ab test is used as a screening test and identifies whether someone has been exposed to the virus or not. The test is usually reactive by six weeks, but it could take up to 12 weeks for the HCV Ab test to become reactive after infection (the window period).
- A non-reactive HCV Ab test indicates that the person has not been exposed to hepatitis C. The exception is if their risk is very recent and they have been tested within the window period before the test becomes positive. In this case, a repeat HCV Ab test should be taken in one to two months.
- A reactive HCV Ab test indicates that the person has been exposed to hepatitis C but it does not tell you:
- how long ago they were exposed, or
- whether the infection has resolved naturally or following treatment, or
- whether they have a chronic infection.
Regardless of whether the infection has resolved or not, the HCV Ab test will remain reactive for life; therefore, if someone has ever had a reactive HCV Ab test, there is no point in repeating this test. The HCV RNA test identifies whether hepatitis C is circulating in the bloodstream and should be taken at least once following a reactive HCV Ab test.
- A non-reactive HCV RNA indicates that HCV is not detected and that the person has cleared the infection either naturally or following successful treatment.
- A reactive HCV RNA test indicates the virus is detected due to either an acute or chronic infection. If hepatitis C has been acquired recently, the RNA test should be repeated in six months to check whether hepatitis C has resolved or whether it persists, indicating chronic infection.
HCV genotype testing: There are seven different strains or genotypes of hepatitis C, of which type 1 and type 3 are the most common in Australia. Prior to the initiation of DAA treatment, hepatitis C genotype testing is required to be taken and documented in the patient's medical record.
Table 5. Interpreting HCV tests
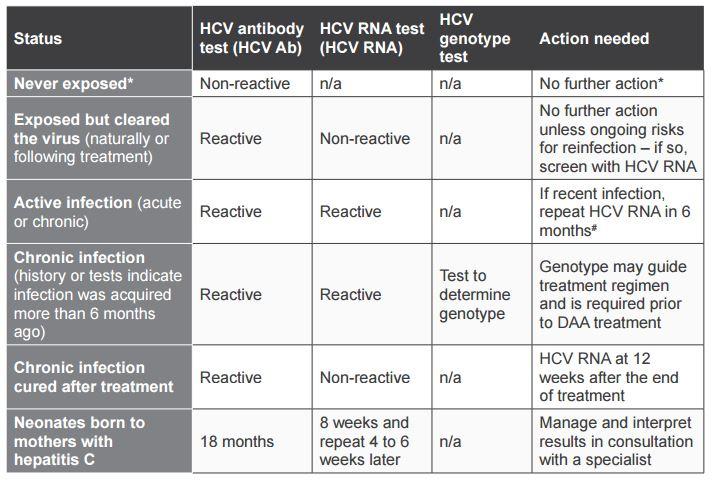
n/a = not applicable /not needed
* unless very recent exposure – repeat test in 1 to 2 months – or with ongoing risk of infection
# At the time of writing, post-exposure prophylaxis and DAA treatment of acute hepatitis C is not recommended. Any updates or changes to current recommendations can be found at the Australian recommendations for the management of hepatitis C infection.
http://www.hepcguidelines.org.au/
Management of hepatitis C
Increasing access to DAA and improving adherence are crucial to enabling the effective treatment and cure of hepatitis C. As treatment regimens with DAA are likely to change over time, detailed information regarding current treatment is not included in this manual but can be found at the Australian Recommendations for the Management of Hepatitis C Virus Infection: http://www.hepcguidelines.org.au/
DAA treatment can be prescribed by medical officers and nurse practitioners through primary health services. Even if not prescribing DAA, it is important for all practitioners to understand the principles of hepatitis C management as they are likely to be involved either directly or indirectly in different aspects of the care of people with hepatitis C. Always ensure information provided is accurate and up-to-date, and given in a way that respects privacy and confidentiality. Also be sensitive to issues such as health literacy.
In addition to DAA treatment, there are many other benefits for people with hepatitis C to engage with primary healthcare services, particularly as some may have complex medical and social needs. Appropriate management not only prevents or limits progression of liver disease but also provides opportunities to manage and support other medical and social issues through primary health or referral to other services.
The following key points are provided as a guide to initial and ongoing management of people with hepatitis C. Further advice and support can be provided through HepatitisWA: http://www.hepatitiswa.com.au/
Initial diagnosis of hepatitis C
- Provide appropriate information to explain what the reactive HCV Ab screening test means.
- Explain the need for further testing with HCV RNA to exclude or confirm chronic infection.
- If chronic infection is confirmed by a reactive HCV RNA, explain what other tests are needed as part of initial management.
- People who are currently injecting drugs should be encouraged to advise their regular injecting partners of their diagnosis, to enable them to access testing and treatment.
Recall, follow-up and ongoing management of hepatitis C
How people will react to the diagnosis of chronic hepatitis C and their knowledge regarding consequences and treatment will vary; therefore, follow-up should be tailored to the needs of individuals. While several consultations may be needed following the initial diagnosis, information that should be discussed includes general information regarding hepatitis C, follow-up and management, and specific information about the availability and benefits of DAA treatment. Facilitate follow-up by gaining consent to recall, clarifying the preferred method for followup and checking contact details are up-to-date. While the decision to commence DAA will be straightforward for many, for others it may take some time. Ensure people are supported throughout this process and provided with appropriate management in the meantime. More information on management and pre-treatment assessment can be found in the Silver Book. www.health.wa.gov.au/Silverbook
Ensure that key information regarding DAA is provided to people with hepatitis C:
- Highlight the benefits of treatment and ensure clients are aware of access to treatment, general principles of treatment regimens and cure rates.
- Address any misconceptions regarding treatment, particularly those that have changed with new DAA treatment such as:
- improved adherence due to single daily dosing, fewer side-effects and shorter treatment times
- liver biopsy no longer required as a criterion for treatment
- current injecting drug use not a barrier to accessing treatment.
DAA can be prescribed by medical practitioners or authorised nurse practitioners experienced in the treatment of hepatitis C, or in consultation with experienced specialist physicians. Further information regarding training and support for practitioners to prescribe DAA can be found on the Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM) website: https://ashm.org.au/
Following the initial diagnosis and before DAA treatment is commenced, people with hepatitis C should have a six-monthly check-up at a minimum. Management should include, but is not limited to, the following:
- physical examination to check for signs of chronic liver disease and cirrhosis
- full blood examination, tests for liver function, alpha-fetoprotein (AFP), urea and electrolytes, and eGFR (estimated glomerular filtration rate, which is an overall measure of kidney function)
- liver fibrosis assessment (which may require liver elastography)
- liver ultrasound among people with cirrhosis to exclude HCC
- provide information regarding key issues such as preventing ongoing transmission and limiting progression to cirrhosis and liver cancer
- offer testing for other BBVs (HIV, hepatitis B) and sexually transmitted infections (STIs) as appropriate
- provide hepatitis A and B immunisation (if not immune)
- encourage and support the reduction of alcohol consumption
- conduct a general or adult health check, if due
- identify and manage co-morbidities such as obesity, diabetes and renal disease
- review concurrent medications (prescription and other drug use)
- provide counselling, support and referral to other services, such as alcohol and other drug services and mental health services, as required
- enable access to peer and social support.
Criteria for treatment of hepatitis C
Virtually all people with hepatitis C are now eligible for treatment, including those who are current injecting drug users who were previously ineligible for treatment or intolerant of interferon. The exceptions to this are that treatment is not recommended for people with limited life expectancy or for women who are pregnant. Pregnancy should also be avoided for six months following treatment. Children under the age of 18 should be managed by a paediatrician experienced in managing hepatitis C.
In addition to clearing the infection, successful treatment also leads to improvements in quality of life, regression of liver fibrosis and cirrhosis, and reduction in the risk of liver failure and liver cancer. Key points with regard to DAA treatment for hepatitis C:
- DAA treatment enables greater than 95 per cent cure rates.
- Some medications are co-formulated to enable single daily dosing.
- Treatment regimens may be guided by the specific HCV genotype or cover most genotypes (pan-genotypic).
- Other factors influencing the choice of treatment regimen include prior treatments, the presence of cirrhosis, drug interactions with other medications, and co-morbidities.
- Treatment is given for an average of 12 weeks (ranging from 8 to 24 weeks).
- Fatigue, headache and nausea are the most common side-effects but are uncommon and typically mild.
- 'Cure' is measured by having a sustained virological response (non-reactive HCV RNA) at 12 weeks following completion of treatment.
- Drug interactions and co-morbidities such as HIV, hepatitis B and renal disease may complicate treatment but not necessarily prevent it.
- Renal function needs to be assessed prior to treatment.
- People with significant renal impairment (eGFR < 50 mL/min/1.73 m2 ), and others with complex co-morbidities, should be referred to or managed in consultation with appropriate specialists.
Less intense monitoring is needed while on DAA treatment; however, follow-up may need to be tailored for individual clients, particularly if there are other medical or social issues that may complicate management or where more support may be needed to ensure adherence.
Treatment failure is defined as a reactive HCV RNA at 12 weeks following the end of treatment. While uncommon, in the event of treatment failure, consult with a specialist regarding ongoing management or referral.
Hepatitis B: testing and treatment
The risk factors and general management of hepatitis B share many similarities to that of hepatitis C, as well as some distinct differences. While DAA is also used in the management of hepatitis B, treatment is ongoing and does not necessarily lead to a cure, but can limit progression to cirrhosis and HCC. Treatment of hepatitis B is not as straightforward as for hepatitis C. It can only be prescribed by specialists and by accredited and approved GP s100 prescribers, and is often managed in conjunction with specialists. All people with chronic hepatitis B, regardless of DAA treatment, should have a regular 6 to 12 monthly check-up with their general practitioner (GP) to check for signs and symptoms of liver disease.
Who and when to test for hepatitis B
Hepatitis B is transmitted via blood and body fluids so that while risk factors are similar to those outlined for hepatitis C, hepatitis B is primarily transmitted from mother to baby, through unsafe sex or unsafe injecting. Aboriginal people, particularly those born before the introduction of universal neonatal vaccines, as well as people born in high prevalence countries, remain at significant risk of infection and progression to cirrhosis and liver cancer.
In contrast to hepatitis C, hepatitis B is a vaccine preventable infection, so ensuring people at risk are adequately immunised is an important public health measure to prevent transmission. All Aboriginal and Torres Strait Islander people and others at risk should be tested at least once to determine their hepatitis B status. If not immune and not infected, immunisation should be encouraged and provided as outlined in the Australian Immunisation Handbook, and their immune status should be appropriately documented. People who are immune, either as a result of a resolved infection or immunisation, do not need to be screened again for hepatitis B. The exception to this is during pregnancy, when all women are tested for hepatitis B at their first visit as part of routine antenatal screening and again in the third trimester, if at high risk.
Interpretation of hepatitis B tests
There are several tests for hepatitis B and three different tests are needed to interpret results appropriately. The following tests should be taken when screening for hepatitis B among people who have never been tested:
- antibody to core antigen (anti-HBc)
- antibody to surface antigen (anti-HBs)
- hepatitis B surface antigen (HBsAg).
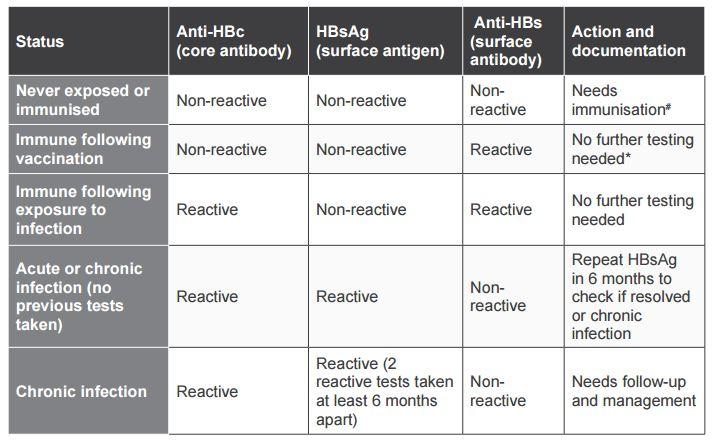
Anti-HBc indicates exposure to hepatitis B:
- A non-reactive test means that the person has not been exposed to hepatitis B. Unless they have had a recent exposure, in which case repeat the test in 1 to 2 months.
- A reactive test means that the person has been exposed to hepatitis B but does not tell you:
- how long ago they were exposed, or
- whether they have a chronic infection, or
- whether they cleared the infection and are now immune.
HBsAg indicates an acute or chronic infection:
- A reactive test means that the person has either an acute or a chronic infection – repeat in six months after an acute infection or if there is no prior history of testing.
- Two reactive HBsAg taken at least six months apart indicate a chronic infection.
- A non-reactive test and a reactive anti-HBc means that the person has cleared the virus naturally following exposure.
The HBsAg test is often the only test taken as part of antenatal screening to identify active infections and prevent mother to child transmission. Be aware that an HBsAg test alone does not tell you whether the person is immune or needs vaccination.
Hepatitis B surface antibodies (anti-HBs) indicate immunity and can be measured following clearance of an acute infection or successful immunisation:
- A reactive test indicates immunity after vaccination or clearance of an infection.
- A non-reactive test means that the person is not immune.
Hepatitis B e antigen (HBeAg) may be routinely tested by the laboratory if HBsAg is reactive. While a reactive HBeAg indicates higher infectivity, a non-reactive test (and reactive anti-HBe) does not mean that the person is not infectious.
Hepatitis B DNA (HBV DNA) measures the amount of virus in the blood and can be used to monitor chronic infection and response to treatment.
Table 6. Interpreting hepatitis B tests
Management of hepatitis B
To prescribe treatment for hepatitis B through primary healthcare services, GPs need to complete the Hepatitis B Community s100 Prescribers Program, which is administered by ASHM and the Department of Health. This program provides initial training and ongoing support such as through access to specialist clinicians working in designated liver clinics and continuing professional development activities. More information on prescribing and training can be found on the ASHM website: https://ashm.org.au/
Regardless of DAA treatment, remember that people with chronic hepatitis B should be monitored regularly (every 6 to 12 months) by their GP for signs and symptoms of liver disease.
Re-engaging people with hepatitis B or C who are lost to follow-up
Enabling access to treatment involves the detection of new cases through increased screening as well as re-engaging people diagnosed in the past who may be unaware of new treatments or who have become lost to follow-up. There are many reasons why people become lost to follow-up and they may be related to both the client and health service:
- Client-related factors:
- lack of awareness of the benefits of new DAA over older treatments
- misconceptions about the criteria for accessing treatment
- other health or social issues taking priority
- change in contact details or place of residence
- not wishing to engage with any health services
- using another health service.
- Health service factors:
- real or perceived issues that create barriers to accessing services (e.g. privacy, acceptability, and fear of being judged or discriminated against)
- recall systems may not be robust enough to prevent loss to follow-up of clients over a long period of time.
Some of these issues may not be easily addressed, but there are many ways in which health services can reduce barriers for people accessing the service and treatment, including:
- ensuring staff provide an environment that is confidential, non-judgemental, non-discriminatory and acceptable and accessible to clients
- supporting staff training to ensure all staff are aware of the benefits of new DAA treatment and criteria for prescribing and accessing treatment through primary healthcare services
- reviewing and strengthening systems for documentation and recall to enable effective management and prevent or limit loss to follow-up
- conduct clinical audits to re-engage with people who may not have had chronic hepatitis C infection confirmed with HCV RNA, who may not be aware of DAA treatment or who have become lost to follow-up.
Health service staff should be familiar with their responsibilities with regard to data entry and health information systems (HIS). Documentation should be clear and consistent, and easily extractable at a later date. In particular, staff should ensure appropriate documentation of hepatitis B and hepatitis C status, follow-up relating to outstanding tests, and management of hepatitis A and hepatitis B immunisation.
Conducting clinical audits to re-engage people lost to follow-up
Conducting clinical audits to identify people with HBV and HCV lost to follow-up can be complicated by the fact that their medical histories may go back many years, data may have been entered in an inconsistent manner and extracting relevant data may not be straightforward. While HIS continue to evolve, making data entry and extraction easier, depending on the size of the service, it may still take a dedicated staff member several days to conduct an audit. However, the time and resources spent have significant health benefits for individuals and cost benefits for health services in the long run.
Some of the information that could be extracted through an audit to assist with follow-up includes the following:
- Among people who have had a reactive HCV Ab test, identify and recall those for HCV RNA testing (if it has not been done) to confirm or exclude chronic infection.
- Identify all people who have had chronic HCV or HBV confirmed, and check whether they are having regular monitoring or are having treatment.
- Identify and recall people with HCV who have either not been treated, or for whom treatment was not successful (indicated by a reactive HCV RNA at follow-up).
- Create and maintain a list of those who do require follow-up and those who do not.
- During the process of conducting an audit, ensure missing or incorrect data is entered accurately in a way that can be extracted easily at a later date.
Clinical audits have already been conducted in some regions and by individual health services. To conduct an audit, seek advice from staff of the health service continuous quality improvement (CQI) program or contact the Aboriginal Health Council of WA (AHCWA) or the regional public health units (PHUs) in regard to whether they are able to provide more information or support. ASHM and Communicare have developed a manual to assist with conducting audits that can be accessed through the ASHM website: https://ashm.org.au/

